Biowaiver Criteria for Multiple Strengths Across Regulatory Bodies
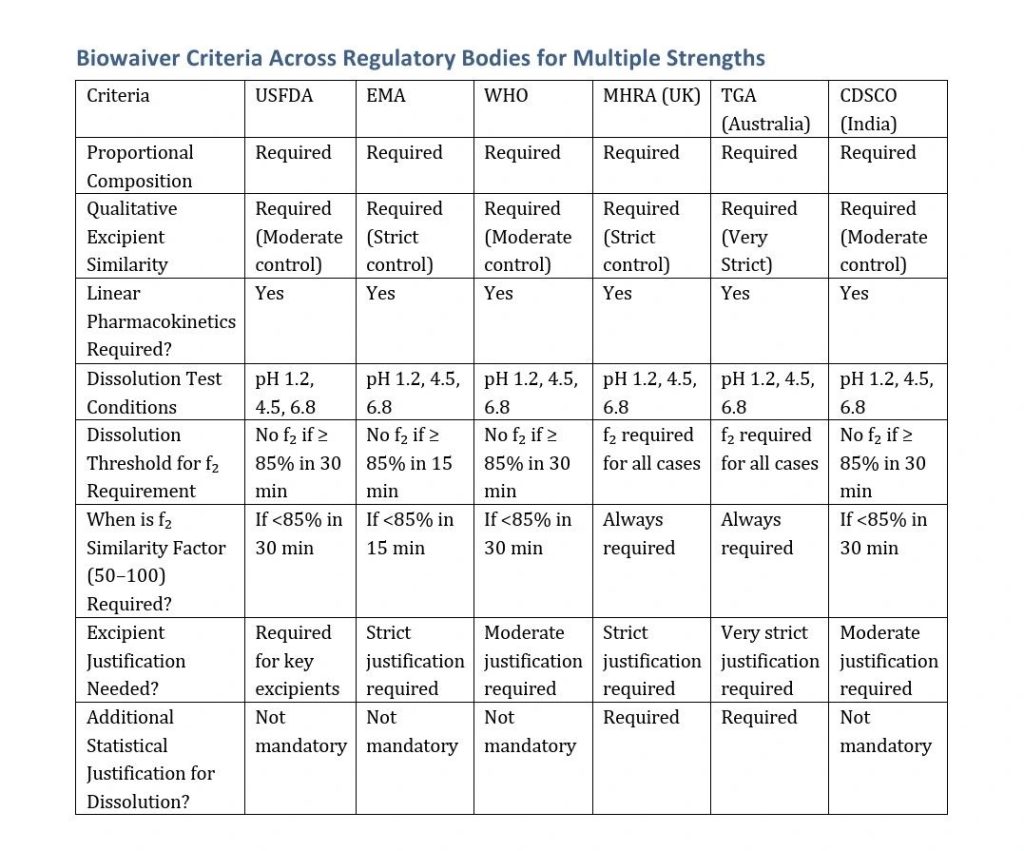
When BE is demonstrated for one strength of a drug product, regulatory agencies allow a biowaiver for additional strengths under certain conditions. The key criteria vary across USFDA, EMA, WHO, MHRA, TGA, and CDSCO but are largely aligned on fundamental principles.
USFDA Biowaiver Criteria
Biowaiver is allowed for additional strengths if:
- The formulation is qualitatively similar across all strengths.
- The additional strengths are dose-proportional to the bio-tested strength.
- The drug exhibits linear pharmacokinetics.
- Dissolution profile criteria:
- If dissolution is ≥ 85% in 30 minutes across three pH media (1.2, 4.5, 6.8), f₂ similarity analysis is NOT required.
- If dissolution is < 85% in 30 minutes, f₂ similarity factor (50–100) is required.
EMA Biowaiver Criteria
Follows a similar approach to USFDA with slight variations:
- Proportional formulation: The ratio of active ingredient to excipients must be similar across strengths.
- Linear pharmacokinetics: Required.
- Dissolution profile criteria:
- If dissolution is ≥ 85% in 15 minutes in three pH media (1.2, 4.5, 6.8), f₂ similarity analysis is NOT required.
- If dissolution is < 85% in 15 minutes, f₂ analysis (50–100) is required.
WHO Biowaiver Criteria
Aligns closely with USFDA and EMA, with the following conditions:
- Qualitative similarity: The formulation must have the same excipients.
- Proportional composition: Minor variations in excipients are accepted.
- Dissolution profile similarity:
- If ≥ 85% dissolution in 30 min, no f₂ required.
- If slower dissolution, f₂ analysis is mandatory.
MHRA (UK) Biowaiver Criteria
Based on EMA guidelines, but stricter excipient control:
- Dissolution profile matching required (similar to EMA).
- Justification required for excipient variations, especially those affecting bioavailability.
TGA (Australia) Biowaiver Criteria
Follows EMA principles but includes:
- Statistical justification for dissolution similarity.
- Strict excipient assessment, requiring justification for changes.
CDSCO (India) Biowaiver Criteria
Follows USFDA/EMA guidelines:
- Proportional formulation required.
- Dissolution profile:
- If ≥ 85% in 30 min, no f₂ needed.
- If < 85%, f₂ analysis is required
Key Takeaways:
- USFDA and EMA: Allow biowaivers without f₂ analysis if dissolution is fast (≥85% release in 30 min for USFDA, 15 min for EMA).
- WHO and CDSCO: Follow USFDA principles.
- MHRA and TGA: More strict, requiring f₂ even if dissolution is rapid.
- TGA and MHRA: Strictest excipient control, requiring additional justification.
- If dissolution is slow (<85% in required time), f₂ similarity (50–100) is required in all agencies.
Read also:
Resource Person: Moinuddin syed. Ph.D, PMP®







