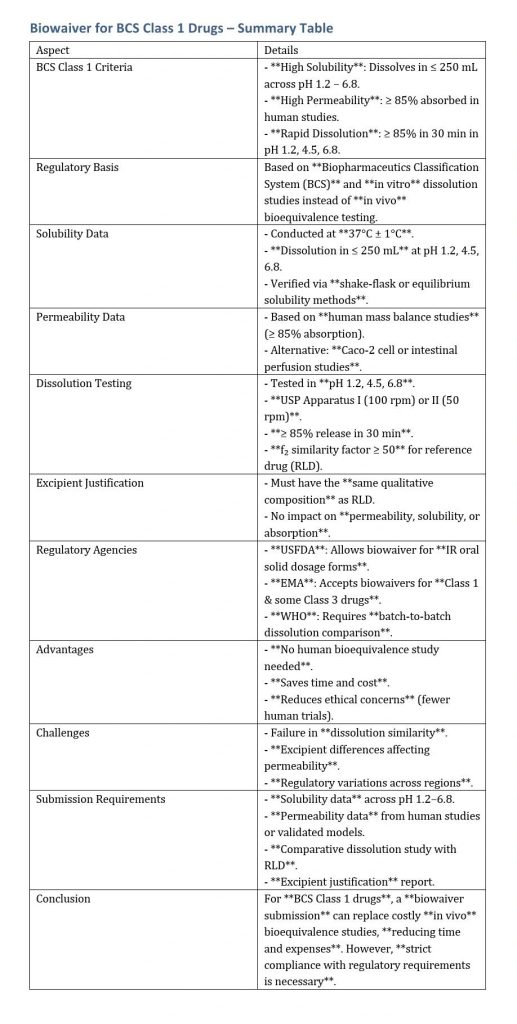
Biowaiver for BCS Class 1 Drugs
A biowaiver allows a generic drug to be approved without an in vivo (BE) study based on in vitro dissolution data. This is particularly applicable BCS Class 1 drugs, which have high solubility and high permeability.
Biowaiver Justification for BCS Class 1 Drugs
To obtain a biowaiver for a BCS Class 1 drug, the following scientific justifications and data must be provided:
A. Solubility Data
Methodology:
- Solubility studies must be conducted at 37°C ± 1°C in aqueous media of pH 1.2, 4.5, and 6.8.
- Use a validated shake-flask or equilibrium solubility method.
- Data should confirm that the drug dissolves completely within the required volume (≤ 250 mL).
Regulatory Requirement:
- USFDA, EMA, and WHO require solubility studies at different pH levels covering the gastrointestinal tract.
- Justification if the solubility is pH-dependent.
B. Permeability Data
Methodology:
- Human mass balance studies showing ≥ 85% absorption.
- In vitro Caco-2 cell permeability studies or intestinal perfusion studies as supportive data.
Regulatory Requirement:
- If human mass balance data is unavailable, permeability studies in validated models (Caco-2, rat intestinal perfusion, etc.) can be used.
- EMA and WHO require evidence from at least two independent permeability studies.
C. Comparative Dissolution Testing
To support a biowaiver, comparative in vitro dissolution testing must demonstrate similarity between the test (generic) product and the (RLD).
Dissolution Media:
- pH 1.2 (acidic)
- pH 4.5 (acetate buffer)
- pH 6.8 (phosphate buffer)
Acceptance Criteria:
- ≥ 85% dissolution within 30 minutes.
- Use of f₂ similarity factor to compare dissolution profiles:
- f₂ ≥ 50 indicates similarity.
- f₂ < 50 requires further justification.
D. Excipients Justification
- The formulation should contain excipients in the same qualitative composition as the RLD.
- Excipient effects on permeability and solubility must be justified.
- Surfactants, solubilizers, and complexing agents may require additional justification.
Regulatory Agencies and Biowaiver Approval
A. USFDA
- Accepts BCS Class 1 biowaivers for immediate-release (IR) oral solid dosage forms.
- Comparative dissolution and excipient justification are required.
B. EMA
- Accepts BCS Class 1 and some Class 3 drugs for biowaivers.
- Requires additional permeability studies if mass balance data is unavailable.
C. WHO
- Requires multimedia dissolution data in three different batches.
- May require additional evidence for global regulatory acceptance.
Common Reasons for Biowaiver Rejection
- Dissolution profile difference between the generic and RLD.
- Formulation differences affecting drug absorption.
- Excipient differences impacting permeability or solubility.
Conclusion
For BCS Class 1 drugs, a biowaiver submission can replace costly in vivo bioequivalence studies, reducing time and expenses. However, strict compliance with regulatory requirements is necessary.
Read also:
Resource Person: Moinuddin syed. Ph.D, PMP®







