Bypassing Patents for Para IV Filing
Filing a Paragraph IV (Para IV) certification for a generic drug requires either proving non-infringement or challenging the validity of the patent covering the reference listed drug (RLD). A deep understanding of independent and dependent claims is crucial to designing around a patent.
Understanding Patent Claims
Patent claims define the legal protection granted to an invention. They can be:
Independent Claims
- Broadest in scope and do not rely on any other claims.
- Define the core inventive concept of the patent.
- Example: “A pharmaceutical composition comprising drug X and an excipient Y.”
Dependent Claims
- Narrower in scope, referring back to an independent claim.
- Add limitations such as dosage strength, formulation method, excipient selection, or particle size.
- Example: “The composition of claim 1, wherein the drug X is present in an amount of 10 mg.”
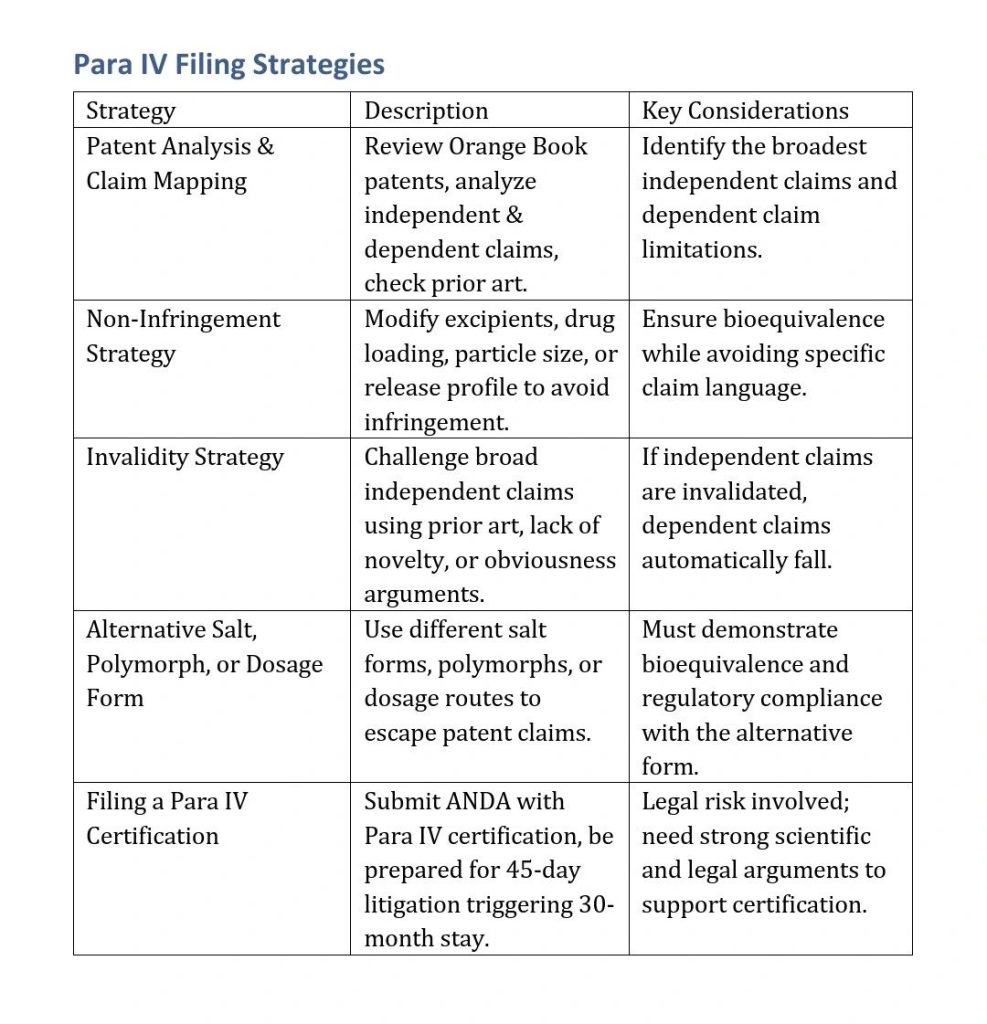
Strategies to Bypass a Patent in Para IV Filing
Patent Analysis & Claim Mapping
- Review the Orange Book patents for formulation, method of use, and crystalline forms.
- Identify independent claims to assess broad patent coverage.
Non-Infringement Strategy
- Reformulate the drug by modifying excipients, drug loading, particle size, or dissolution profile.
- Design around dependent claims by changing dosage strength, release mechanism, or manufacturing process.
Invalidity Strategy
- Conduct a prior art search to find evidence that the patent is obvious or lacks novelty.
- Challenge broad independent claims, as invalidating them can render dependent claims unenforceable.
Alternative Salt, Polymorph, or Dosage Form
- Utilize a different crystalline form, salt, or polymorph to escape claim coverage.
- Change route of administration (e.g., tablet → suspension) to avoid infringement.
File a Para IV Certification
- Declare that the generic formulation does not infringe or that the patent is invalid.
- Be prepared for litigation within 45 days, leading to a 30-month stay unless the case is won.
Read also:
- Paragraph IV (Para IV) Filing | Overview & Strategies
- Intellectual Properties Rights in Pharma Industry
- Understanding Patent Length and Generic Competition in Pharmaceuticals
Resource Person: Moinuddin syed. Ph.D, PMP®


