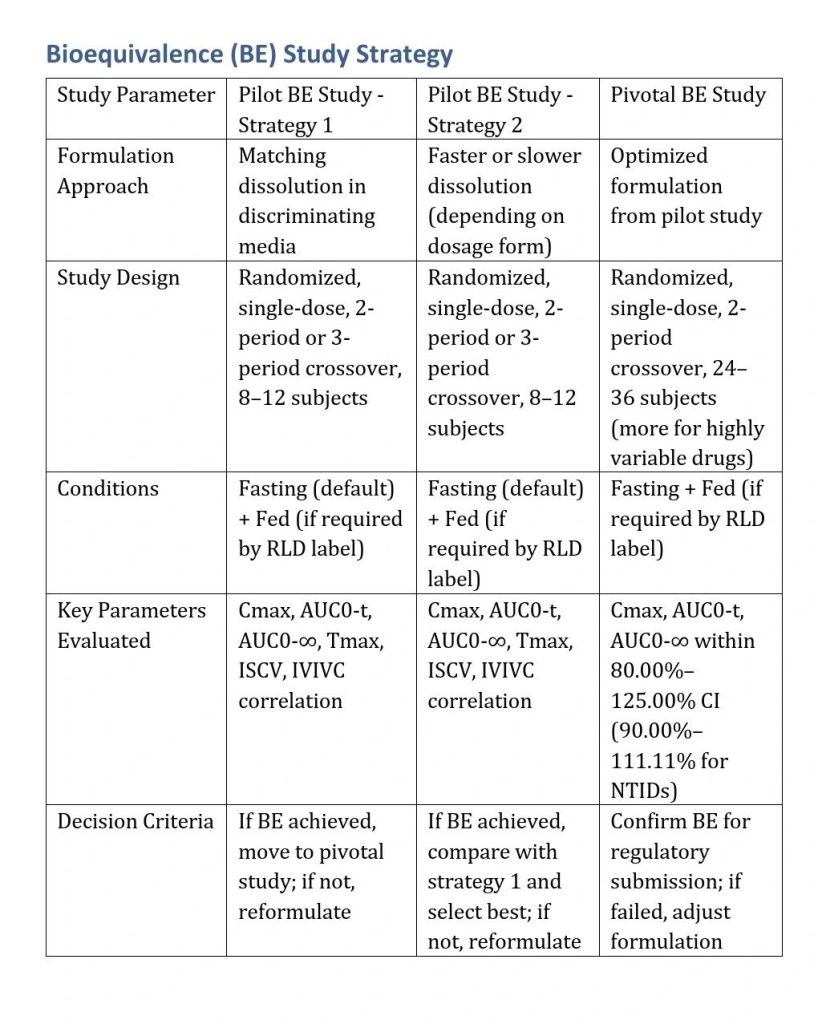
Pilot and Pivotal Bioequivalence (BE) Study Strategy for Generics
Pilot BE Study with Two Dosing Strategies
To optimize the formulation before the pivotal BE study, two pilot BE studies are conducted with different dissolution behaviors:
Strategy 1: Formulation with Matching Dissolution in Discriminating Media
- Ensures that the test product mimics the reference product dissolution in a discriminating medium.
- Helps assess whether dissolution similarity translates into in vivo bioequivalence.
Strategy 2: Formulation with Faster or Slower Dissolution (Depending on Dosage Form)
- Faster Dissolution: Used for drugs where absorption is dissolution-limited or intended for immediate-release dosage forms.
- Slower Dissolution: Considered for drugs with controlled-release or those exhibiting precipitation/recrystallization risks in vivo.
Pilot BE Study Strategy
Study Design
- Randomized, single-dose, 2-period or 3-period crossover
- Small sample size (8–12 healthy volunteers)
- Fasting condition (default) + Fed condition if required by RLD label
Dosing Strategies:
- First Group: Test formulation with matching dissolution (reference-based)
- Second Group: Test formulation with a different dissolution profile (faster or slower)
- Reference Product (RLD)
Key Parameters Evaluated
- Cmax, AUC0-t, AUC0-∞ (Primary PK parameters)
- Tmax (Absorption rate indicator)
- Intra-subject variability (ISCV)
- Correlation of dissolution vs. in vivo performance
Decision-Making Based on Results
- If both formulations show bioequivalence: Proceed with the one having better manufacturability and stability.
- If the matching dissolution formulation succeeds: Confirms in vitro-in vivo correlation (IVIVC), proceed with pivotal BE study.
- If the faster/slower dissolution formulation performs better: Adjust formulation accordingly and retest if needed.
- If both fail: Reformulate and conduct another pilot BE study.
Pivotal BE Study Strategy
Once the pilot study confirms an optimized formulation, a pivotal BE study is conducted for regulatory submission.
Study Design
- Randomized, single-dose, 2-period crossover study
- Larger sample size (24–36 volunteers, or more for highly variable drugs)
- Fed BE study if required by RLD label
- Replicate study design for highly variable drugs (ISCV > 30%)
- Parallel design for long half-life drugs (t½ > 24h)
BE Criteria
- Cmax, AUC0-t, and AUC0-∞ within 80.00%–125.00% confidence interval (CI)
- For NTIDs: 90.00%–111.11% CI
- Highly variable drugs: Scaled bioequivalence approach if needed
Strategic Advantages of This Approach
- Ensures optimal formulation selection before the pivotal study.
- Reduces risk of BE study failure by evaluating multiple dissolution profiles.
- Helps establish IVIVC, aiding future formulation modifications.
- Regulatory risk minimization by selecting the best formulation upfront.
Read also:
- Factors Affecting Bioequivalence (BE) Results
- Regulatory Approaches for Bioequivalence Study of Highly Variable Drugs (HvD)
Resource Person: Moinuddin syed. Ph.D, PMP®