Biowaivers for Fixed-Dose Combination (FDC) Drug Products
Fixed-dose combination (FDC) drug products present unique challenges in biowaiver assessments, as they involve multiple active pharmaceutical ingredients (APIs) with different physicochemical properties and release characteristics. Regulatory guidelines (USFDA, EMA, WHO) provide biowaiver provisions, but their applicability depends on several factors, such as API solubility, permeability, formulation type, and release mechanisms.
Key Considerations for Biowaivers in FDCs
BCS Classification of APIs
- Biowaivers are more likely if both APIs belong to BCS Class I (high solubility, high permeability) or Class III (high solubility, low permeability).
- If one API falls into BCS Class II or IV, a biowaiver becomes challenging due to solubility or permeability limitations.
Formulation Design & API Distribution
Single-Layer (Immediate-Release, Both APIs in One Layer)
- If APIs are co-formulated in a single layer and the dissolution profile is rapid/similar across pH conditions, biowaivers may be considered.
- Dissolution testing must demonstrate similar release across media (pH 1.2, 4.5, and 6.8) and comparative dissolution with RLD.
Multi-Layer Tablet (One API in Core, One in Coating)
- When one API is in the core and the other in the coating, different release kinetics may occur.
- If the coating dissolves rapidly, it may be treated similarly to an immediate-release form.
- If the coating modifies the release, an in vivo bioequivalence (BE) study is likely needed.
- Dissolution similarity (f₂ test) must be assessed independently for each layer/API.
Modified-Release (One API IR, One API ER/DR)
- When one API follows an immediate-release (IR) profile and the other is extended-release (ER) or delayed-release (DR), a biowaiver is unlikely.
- The ER component generally requires bioequivalence (BE) studies, while the IR component may qualify for a biowaiver under certain conditions.
Regulatory Perspective on Biowaivers for FDCs
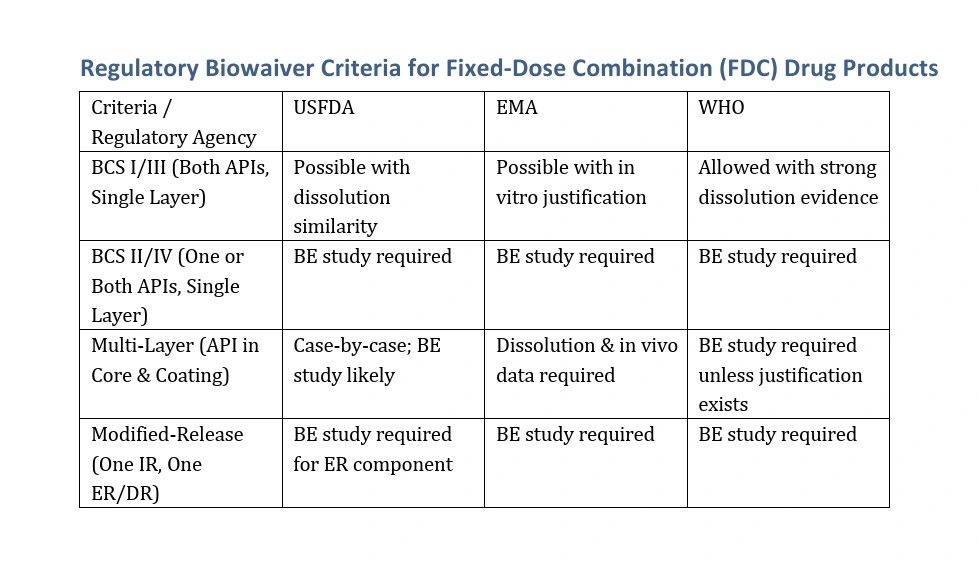
- USFDA: Generally requires BE studies unless both APIs qualify under the BCS-based biowaiver approach.
- EMA: Allows BCS-based biowaivers but emphasizes dissolution and in vitro performance in multi-layered tablets.
- WHO: Recommends in vitro dissolution similarity but often mandates BE studies for FDCs unless strong justification exists.
Conclusion
- Single-layer FDCs with BCS I/III APIs may qualify for a biowaiver.
- Multi-layer formulations, especially with modified-release APIs, often require BE studies.
- In vitro dissolution testing is critical for establishing biowaiver eligibility.
- Regulatory bodies assess each case individually, considering formulation complexity.
Read also:
- Biowaiver for Topical Dosage Forms
- Biowaiver Eligibility for Injectable Formulations
- Biowaiver Considerations for Sustained-Release (SR) Dosage Forms
Resource Person: Moinuddin syed. Ph.D, PMP®