Is a Bioequivalence (BE) Study Needed When Replacing Crospovidone with Croscarmellose?
A BE study may or may not be required, depending on several factors. Here’s a structured approach to determine whether regulatory agencies like the FDA or EMA would require a BE study for this type of qualitative formulation change:
Regulatory Perspective on Excipients in Bioequivalence
Regulatory agencies consider excipients when evaluating bioequivalence, especially when they affect drug release, absorption, or solubility.
Key points for consideration:
- Crospovidone and Croscarmellose Sodium are both super-disintegrants, but they have different properties:
- Crospovidone (PVPP): Works via capillary action and swelling, but does not form a gel.
- Croscarmellose Sodium (CCS): Swells significantly and forms a gel-like structure, which can affect dissolution.
- The switch may alter disintegration time, dissolution rate, and ultimately bioavailability.
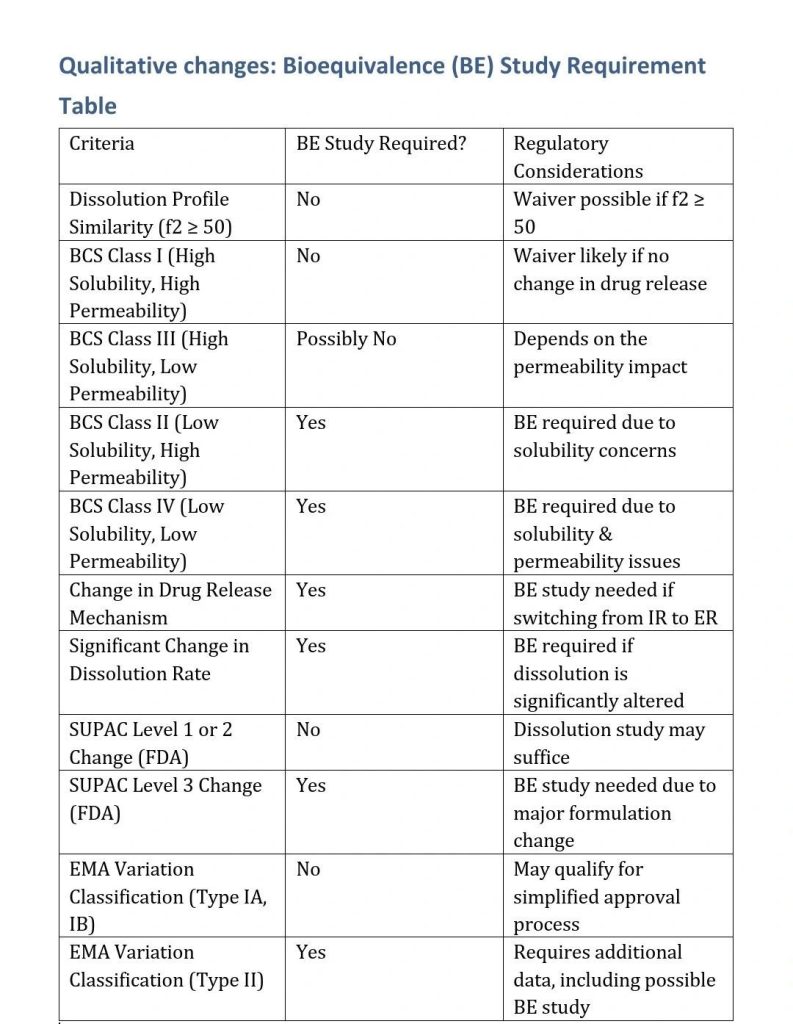
When a BE Study is NOT Required?
Regulatory agencies may waive a BE study if:
- The dissolution profile remains similar (f2 similarity factor ≥ 50).
- The change does not impact drug solubility or permeability (important for BCS Class I & III drugs).
- The drug is a Biopharmaceutics Classification System (BCS) Class I drug (high solubility, high permeability).
- There are no changes in drug release mechanism (e.g., switching from IR to ER would require a study).
- The formulation meets the SUPAC (Scale-Up and Post-Approval Changes) guidelines for Level 1 or Level 2 changes (for the US FDA).
When a BE Study is Required?
A BE study is likely needed if:
- The drug is BCS Class II or IV (low solubility, permeability concerns).
- The dissolution test shows significant differences between the old and new formulation.
- The change significantly affects the disintegration or dissolution rate, impacting bioavailability.
- The regulatory authority specifically requires a BE study due to past precedent or case-by-case evaluation.
Subsequent Steps for Conducting Comparative Dissolution Testing:
- Perform dissolution studies in at least three media (pH 1.2, 4.5, 6.8).
- Calculate the f2 similarity factor (if f2 ≥ 50, dissolution profiles are similar).
Evaluate BCS Class & Drug Release Mechanism:
- If the drug is BCS Class I or III, a waiver may be possible.
- If Class II or IV, regulators will likely require BE data.
Refer to SUPAC (FDA) or Variation Guidelines (EMA):
- For the FDA, this would be a SUPAC-IR Level 2 or 3 change, where dissolution testing may suffice.
- For EMA, check the variation classification (Type IA, IB, or II).
Conclusion
- If dissolution is similar, a waiver is possible (especially for BCS Class I & III drugs).
- If dissolution differs, a BE study is likely required.
- For BCS Class II or IV drugs, be prepared for BE requirements.
- Check regulatory guidelines (FDA SUPAC or EMA variations) for country-specific decisions.
Read also:
- Steady-State Bioequivalence (BE) Studies
- Factors Affecting Bioequivalence (BE) Results
- Paragraph IV (Para IV) Filing | Overview & Strategies
Resource Person: Moinuddin syed. Ph.D, PMP®







