Osmotic Pump Extended-Release Technology for Oral Dosage Forms
Oral drug delivery remains the most convenient and preferred route of administration. However, conventional oral dosage forms often face challenges such as fluctuating drug plasma levels and poor patient compliance. Extended-release formulations, particularly osmotic pump systems, have been developed to overcome these limitations by providing a consistent and controlled release of the drug.
Osmotic pump extended-release technology is a sophisticated drug delivery system designed to provide consistent and controlled drug release over an extended period. This review explores the principles, mechanisms, types, advantages, and applications of osmotic pump systems in oral dosage forms. It also discusses current advancements, challenges, and future directions in this field.
Principles of Osmotic Pump Technology
Osmotic pump systems utilize osmotic pressure to achieve a controlled release of the drug. The core of the system typically contains an osmotic agent and the active pharmaceutical ingredient (API). When exposed to gastrointestinal fluids, water enters the core through a semipermeable membrane, creating osmotic pressure that drives the drug out through a delivery orifice.
Mechanisms of Drug Release
The drug release rate from osmotic pump systems is governed by several factors:
- Osmotic Pressure Gradient: The difference in osmotic pressure between the inside of the pump and the external environment.
- Membrane Permeability: The rate at which water permeates the semipermeable membrane.
- Delivery Orifice Size: The size of the orifice through which the drug is released.
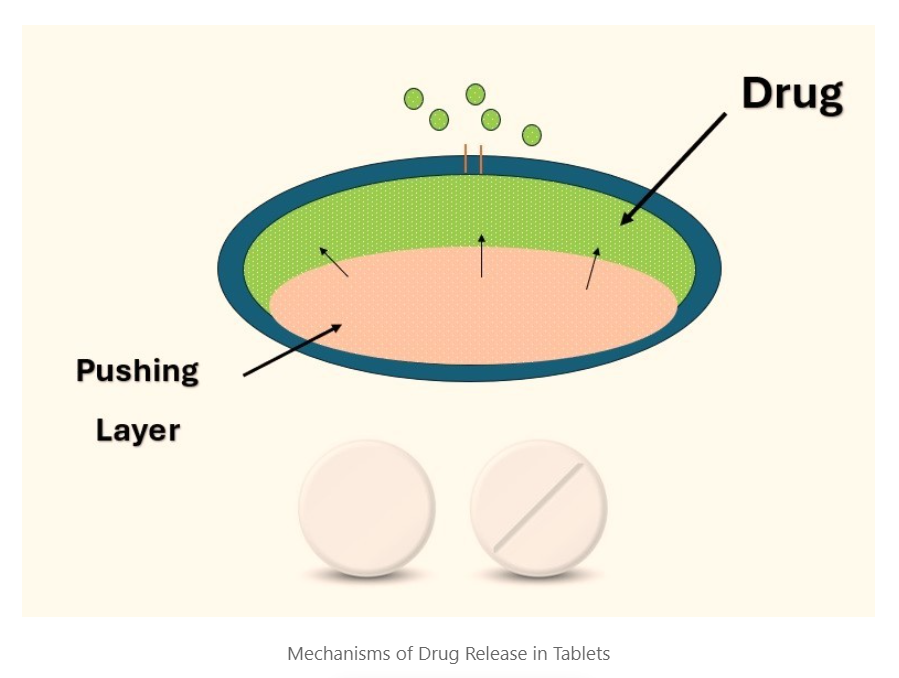
Types of Osmotic Pump Systems
- Elementary Osmotic Pump (EOP): Simplest form, consisting of a core with the drug and osmotic agent, surrounded by a semipermeable membrane with a delivery orifice.
- Push-Pull Osmotic Pump (PPOP): Contains two layers – a drug layer and a push layer. The push layer expands upon water absorption, pushing the drug out.
- Controlled Porosity Osmotic Pump (CPOP): Features a semipermeable membrane with controlled porosity, allowing for more predictable and uniform drug release.
Advantages of Osmotic Pump Systems
- Consistent Drug Release: Provides a zero-order release profile, maintaining consistent drug plasma levels.
- Reduced Dosing Frequency: Enhances patient compliance by reducing the need for frequent dosing.
- Minimized Side Effects: Avoids peak-trough fluctuations, potentially reducing side effects.
- Versatility: Suitable for a wide range of drugs, including those with poor solubility or stability issues.
Applications
Osmotic pump systems are employed in various therapeutic areas, including:
- Cardiovascular Diseases: E.g., nifedipine extended-release tablets for hypertension.
- Diabetes Management: E.g., glipizide extended-release tablets for glycemic control.
- Central Nervous System Disorders: E.g., methylphenidate extended-release tablets for ADHD.
Advancements in Osmotic Pump Technology
Recent innovations include the development of miniaturized osmotic pumps for precise drug delivery, combination systems for multi-drug therapy, and the integration of biodegradable materials for environmentally friendly designs .
Challenges and Future Directions
Despite its advantages, osmotic pump technology faces challenges such as manufacturing complexity, high production costs, and potential for dose dumping. Future research should focus on improving the scalability and cost-effectiveness of these systems, exploring novel materials, and expanding their application to a broader range of drugs .
Conclusion
Osmotic pump extended-release technology represents a significant advancement in oral drug delivery, offering controlled and consistent drug release. Continued research and innovation in this field hold promise for enhancing therapeutic outcomes and patient compliance.
Read also:
- Role of Lipophilicity in Drug Formulation Development
- Bioequivalence Studies for Solid Oral Drug Products
- Formulation Development Strategy for BCS Class IV Molecules
References
- Santus, G., & Baker, R. W. (1995). Osmotic drug delivery: a review of the patent literature. Journal of Controlled Release, 35(1), 1-21.
- Verma, R. K., & Garg, S. (2001). Development and evaluation of osmotically controlled oral drug delivery system of glipizide. European Journal of Pharmaceutics and Biopharmaceutics, 57(3), 513-525.
- Thombre, A. G. (2004). Assessment of the feasibility of oral controlled release in developing world: a case study. International Journal of Pharmaceutics, 272(1-2), 1-13.
- Zentner, G. M., Rork, G. S., Himmelstein, K. J. (1985). Osmotic drug delivery systems: Design and development. Journal of Controlled Release, 1(4), 269-282.
- Liu, L., & Ku, J. (2008). Design and development of a push-pull osmotic pump tablet system with high dosage of acetaminophen. Journal of Controlled Release, 126(1), 45-51.
- Zentner, G. M., Rork, G. S., Himmelstein, K. J. (1991). Controlled porosity osmotic pump. US Patent No. 5,034,229.
- Chien, Y. W. (1992). Novel drug delivery systems. Drugs and the Pharmaceutical Sciences, 50.
- Verma, R. K., & Mishra, B. (1999). Studies on formulation and evaluation of osmotic pump tablets of diltiazem hydrochloride. Drug Development and Industrial Pharmacy, 25(11), 1285-1290.
- Liu, L., & Xu, X. (2008). Preparation of bilayer-core osmotic pump tablets with high release rate and zero-order release of diltiazem hydrochloride. European Journal of Pharmaceutics and Biopharmaceutics, 69(1), 132-140.
- Malaterre, V., Ogorka, J., Loggia, N., & Gurny, R. (2009). Oral osmotically driven systems: 30 years of development and clinical use. European Journal of Pharmaceutics and Biopharmaceutics, 73(3), 311-323.
- Verma, R. K., & Garg, S. (2000). Development and evaluation of osmotically controlled oral drug delivery system of glipizide. European Journal of Pharmaceutics and Biopharmaceutics, 57(3), 513-525.
- Zentner, G. M., Rork, G. S., Himmelstein, K. J. (1991). Controlled porosity osmotic pump. US Patent No. 5,034,229.
- Thombre, A. G. (2004). Assessment of the feasibility of oral controlled release in developing world: a case study. International Journal of Pharmaceutics, 272(1-2), 1-13.
- Theeuwes, F. (1983). Elementary osmotic pump. US Patent No. 4,111,202.
- Liu, L., & Ku, J. (2008). Design and development of a push-pull osmotic pump tablet system with high dosage of acetaminophen. Journal of Controlled Release, 126(1), 45-51.
- Malaterre, V., Ogorka, J., Loggia, N., & Gurny, R. (2009). Oral osmotically driven systems: 30 years of development and clinical use. European Journal of Pharmaceutics and Biopharmaceutics, 73(3), 311-323.
- Li, Y., Zhu, L., Liu, Y., Gao, X., & Jiang, Y. (2010). Design and evaluation of controlled porosity osmotic pump tablets of a highly water-soluble drug chlorpheniramine maleate. Asian Journal of Pharmaceutical Sciences, 5(1), 45-52.
- Gupta, R. B., & Kompella, U. B. (2006). Nanoparticle Technology for Drug Delivery. Drugs and the Pharmaceutical Sciences, 159.
- Gupta, S. K., & Choudhury, P. K. (2001). Controlled porosity osmotic pump tablets of acetazolamide. Pharmaceutical Development and Technology, 6(4), 548-556.
- Thombre, A. G. (2004). Assessment of the feasibility of oral controlled release in developing world: a case study. International Journal of Pharmaceutics, 272(1-2), 1-13.
Resource Person: Mohanned Jallad


